( Until it’s possible. )
Science & Technology
Our Approach
The challenge is simple to state, but demanding in practice: pause molecular motion in every cell of an organ, then safely resume it. To do so, we engineer across five dimensions: perfusion, the cryoprotectants themselves, cooling, rewarming, and assaying organ health. Each breakthrough at the organ scale teaches us how to orchestrate these processes at larger, more complex levels.
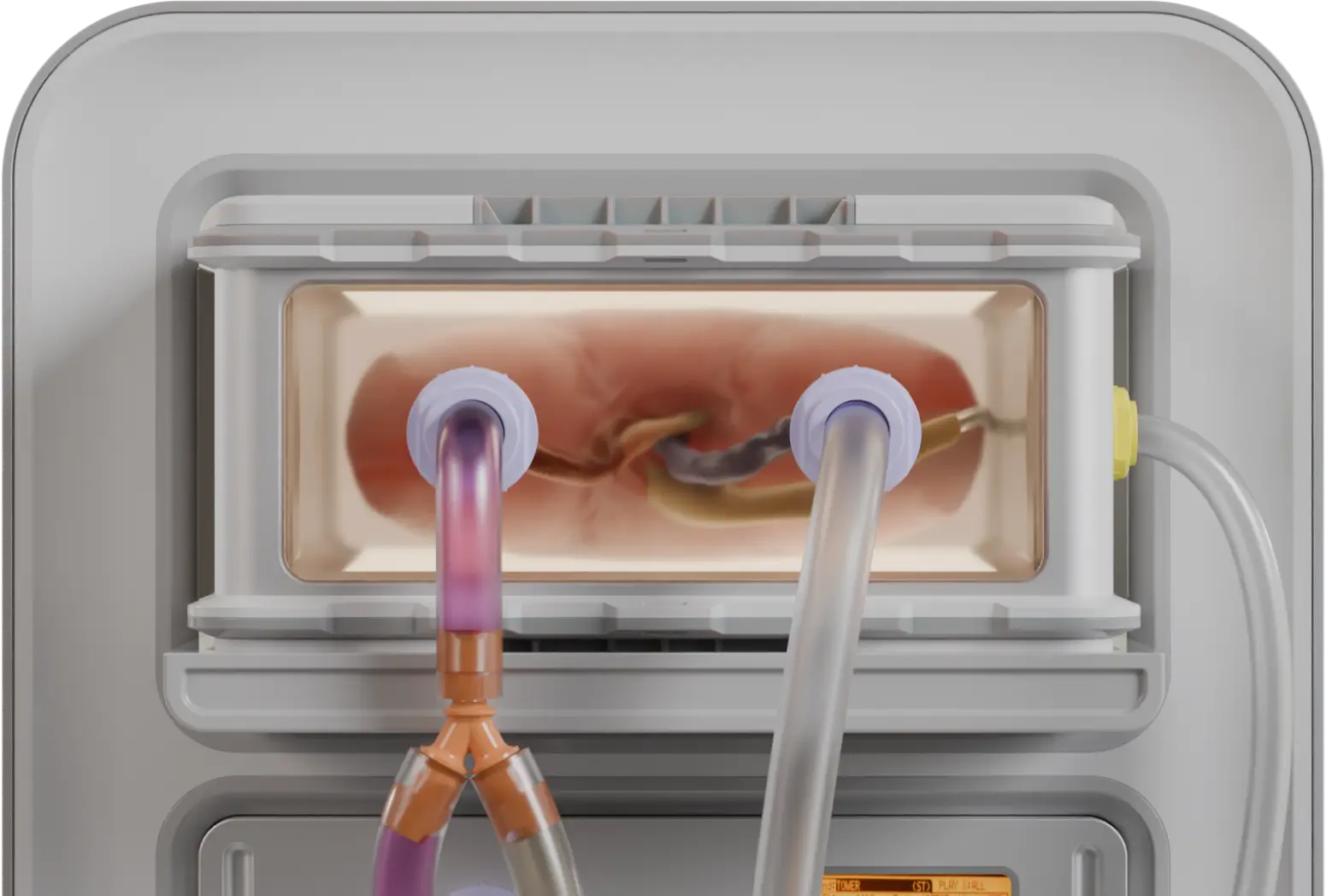

Perfusion
We repurpose biology’s own delivery network.
Perfusion is the process of flowing fluid through a biological system, using pressure, timing, and vascular access to deliver molecules deep into tissue.
Organs are, by necessity, intricately vascularized, a dense map of arteries, veins, and capillaries; no cell lies very far from a blood vessel. Biology evolved this to deliver oxygen and nutrients. We repurpose it to deliver cryoprotectants, nanoparticles, and more time.
The challenge is not simply access to the delicate vasculature, but also control. We develop perfusion protocols that are fast enough to prevent ice, slow enough to avoid osmotic shock, and uniform enough to reach every cell without harm.
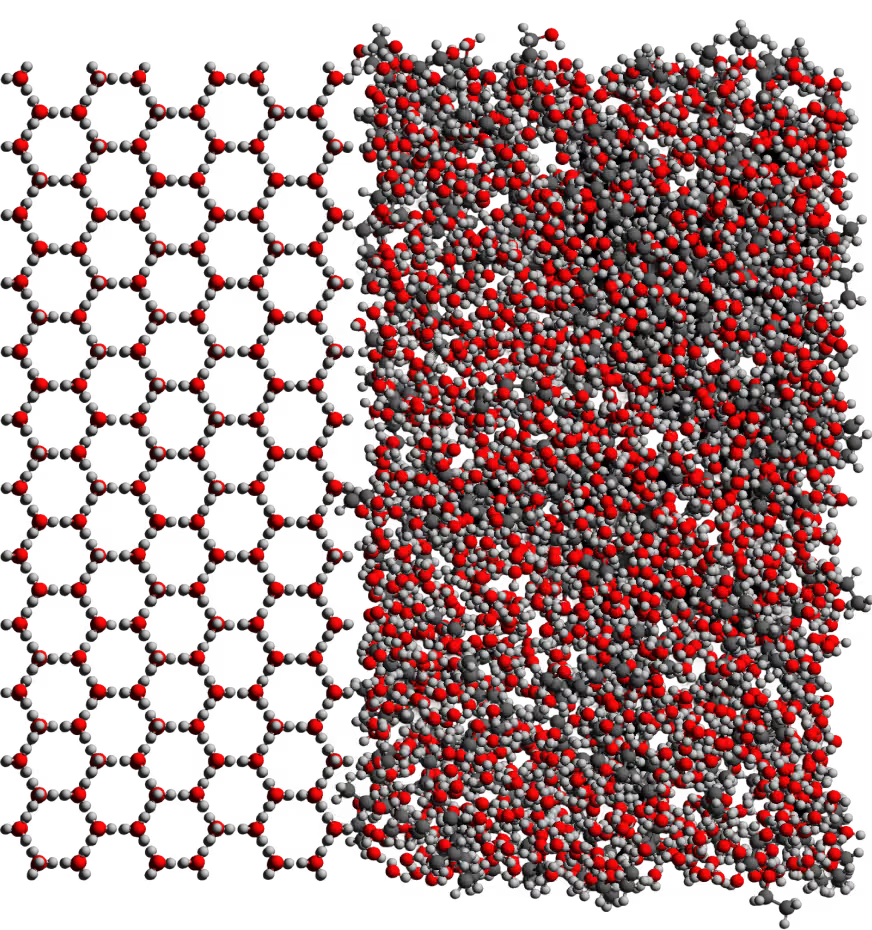
Cryoprotectants
This work traverses theory, atoms, and cells.
Cryoprotectants are small molecules that help biological systems avoid ice formation during cooling. They disrupt hydrogen bonding between water molecules, increase viscosity, and lower the freezing point, allowing tissue to enter a glassy state instead of forming destructive crystals. But these molecules are both protective and perilous. Reaching concentrations high enough to prevent ice often pushes into toxic territory. Load them too slowly, and the tissue will deteriorate from ischemia. Load them too quickly, and cells suffer osmotic shock. Designing safe, effective protocols means finding the narrow path between these risks.
We approach the problem at two scales: atomic and cellular.
At the atomic level, we use Molecular Dynamics Simulations and Machine Learning to model cryoprotectants in silico, predicting how they move, bond, and interact with water.
At the cellular level, we run high-throughput toxicity screens using liquid-handling robots, testing hundreds of candidate cocktails across varying doses and exposure times. The goal is simple but demanding: create cryoprotectant mixtures that are as kind to cells as they are hostile to ice.
Cooling
We don’t freeze. We vitrify.
To preserve an organ, we must bring it below –130°C, where molecular motion halts and time, effectively, stops. But we can’t get there directly. Between 0°C and –130°C lies the danger zone, a window where ice nucleates and extends, tearing tissue apart.
Cooling must be fast enough to pass through this zone before ice forms, but not so fast that the tissue cracks from internal stress. In small systems, surface cooling is enough. But in larger system, surface cooling may create lethal thermal gradients.
We’re building technologies that enable cold to travel deeper, faster, and more evenly.
Rewarming
The return must be as precise as the pause.
Ice has more opportunity to grow on the way up, so we must warm even faster than we cool. But large volumes resist fast, uniform heating. If the surface warms before the core, thermal gradients create stress, and stress creates fractures.
We explore many avenues to solve this. Conventional methods, like convective or microwave heating, often fail at scale. Volumetric rewarming holds more promise. Our engineering team works closely with our perfusionists to deliver rewarming rates with spatial and temporal uniformity.


Assaying Organ Health
Preserving an organ isn’t enough.
Once an organ is rewarmed, we need to know if it’s ready. Clinicians already use standard tools to assess transplant viability, we build on those foundations and adapt them to assess health after cryopreservation.
For example, kidneys are already assessed after normothermic perfusion using trusted clinical markers, like urine production, creatinine clearance, vascular resistance, and injury biomarkers. This gives transplant teams a real-time picture of organ viability.
But cryopreservation changes the baseline. We’re developing new assays to capture what existing metrics can’t: subtle molecular signatures of damage or resilience that emerge only after deep cold. This includes detecting ischemic stress, delayed graft function risk, and cellular recovery trajectories.
We're building upon clinical standards, because preserving an organ isn’t enough. We need to know it’s ready to function, heal, and help.
De-risking whole body
Whole-body cryopreservation is often imagined as science fiction. We see it as a long-term clinical goal made tractable by solving real problems, one biological system at a time. Every breakthrough in organ-scale preservation teaches us how to preserve larger, more complex systems. Perfusion, cooling, rewarming, and assaying — the same principles apply, just with greater orchestration.
But scaling to whole-body preservation introduces new challenges: non-vascular tissues, non-uniform cooling, and above all, the brain. Each demands new architectures of control and a deeper integration of biology, physics, and engineering. We don’t claim it works. Yet. But the barriers are technical, and we have traction.
NeuroscienceTo preserve what matters most.
The Neuroscience and Whole-Body Hibernation team ask the hardest question in cryopreservation: can we pause not just isolated organs, but entire organisms.

NeuroscienceTo preserve what matters most.
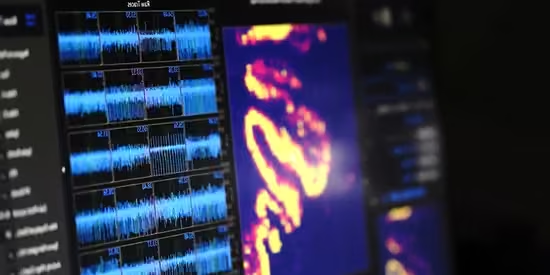
In 2024, we recovered action potentials from cryopreserved and rewarmed mammalian brain tissue — the first time this has been shown in acutely resected neural slices. It's a small step, but a necessary one.
NeuroscienceTo preserve what matters most.
Our team now works to extend that signal: toward synaptic integrity, long-term potentiation, and eventually, whole-brain preservation. We design new cryoprotectants that cross the blood-brain barrier, build perfusion protocols for non-vascular tissue, and develop the assays that can meaningfully measure recovery.
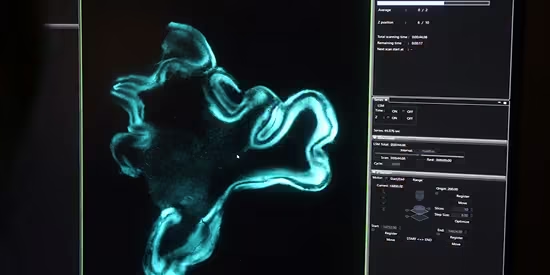
This is the most speculative part of our ambition, and it is the most profound. To work here is to join Until’s most experimental frontier.
Join Us

Until science catches up to need.



We have a world-class team working to make this a reality.